Table of Contents
This pH Calculator makes science easy and fun. Just type in a few details, and it quickly shows how acidic (pH) or basic (pOH) your liquid is. It also tells you the amounts of hydrogen ions ([H⁺]) and hydroxide ions ([OH⁻]) inside.
Whether it’s a strong or weak acid or base, the tool gives you fast, correct results in seconds. No hard math, no confusing formulas, just simple, clear answers that help you learn how chemistry works.
How to Use the pH Calculator
Using this pH calculator is quick, intuitive, and requires no manual computation. Whether you’re a student, lab technician, or simply curious, this tool simplifies the entire process of calculating pH based on your input. Just follow these steps:
1. Calculate pH from the Concentration of an Acid
If you know the molar concentration of an acid, select this option.
- Choose your acid from the built-in list of common strong and weak acids, such as HCl, HNO₃, or CH₃COOH.
- Enter the concentration in mol/L (M).
- The tool instantly calculates pH, [H⁺], [OH⁻], and pOH.
For acids not listed, choose Custom. You’ll then enter the acid’s ionization constant (Ka) and concentration. The tool uses this data to determine the degree of dissociation and outputs the accurate pH.
2. Calculate pH from the Concentration of a Base
This option mirrors the acid method, but it’s tailored for alkaline compounds.
- Pick a base like NaOH or NH₃ from the dropdown.
- Input the molar concentration.
- You’ll receive immediate values for pH, pOH, [H⁺], and [OH⁻].
If your base isn’t listed, select Custom and enter the base dissociation constant (Kb) along with concentration. The pH will be computed through equilibrium calculations.
3. Calculate pH from the Mass and Volume of an Acid
Choose this option when you have a solid acid and need to find the solution pH.
- Select the acid from the list.
- Input the mass (grams, mg, etc.) and the volume of solution (mL, L, etc.).
- The calculator converts the mass into molarity internally and provides full pH results.
For unlisted acids, select Custom and provide the Ka value alongside your mass and volume. The tool applies mole-based equilibrium principles to generate accurate values.
4. Calculate pH from the Mass and Volume of a Base
The same process applies here, but for solid bases.
- Select your base from the dropdown.
- Input the mass and solution volume.
- The tool calculates molarity, then uses the appropriate equations to give you pH, [OH⁻], and pOH.
For custom bases, enter the Kb value and let the calculator handle the chemistry.
5. Calculate pH from Ion Concentration
This is the most direct method if you already know ion values.
- Choose from:
a. Hydrogen ion concentration [H⁺]
b. Hydroxide ion concentration [OH⁻]
c. pOH value
Input one, and the calculator will determine all the rest, pH, pOH, [H⁺], and [OH⁻], using water’s ion product constant Kw = 1 × 10⁻¹⁴ at 25°C.
This calculator also displays additional data like pKa, ion concentrations, and equilibrium constants where applicable. It’s ideal for students, chemists, or anyone needing fast, accurate answers on how to calculate pH from any starting point.
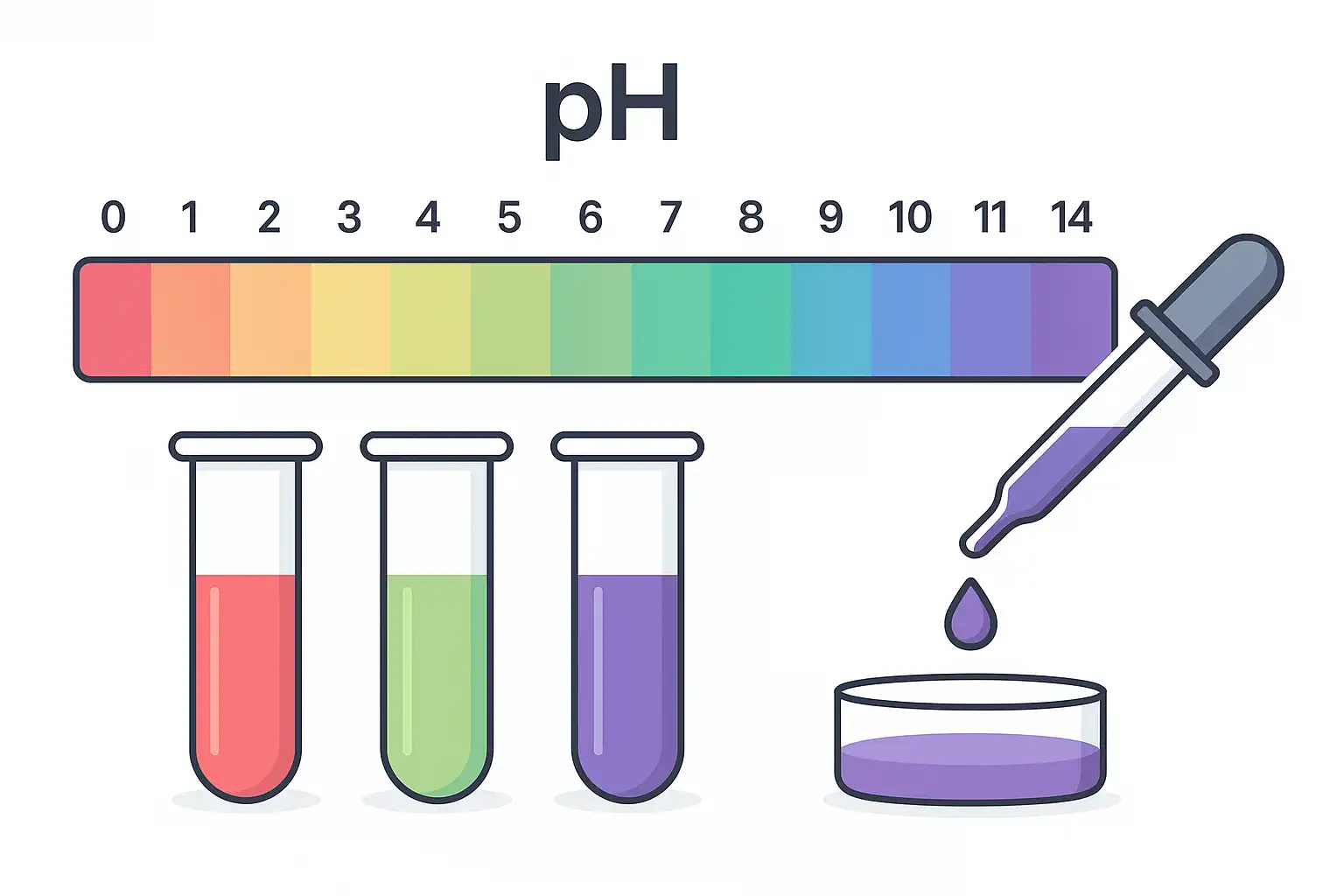
How Does the pH Scale Work?
The pH scale is a numeric system that indicates how acidic or basic (alkaline) a solution is, based on its hydrogen ion concentration [H+][H⁺][H+]. According to the U.S. Geological Survey, the scale ranges from 0 to 14, where 0 represents the most acidic condition, 14 the most basic, and 7 is neutral, as in the case of pure water.
Each step on the pH scale reflects a tenfold difference in hydrogen ion concentration. That means a substance with a pH of 3 is ten times more acidic than one with a pH of 4. This logarithmic nature makes the scale especially useful in disciplines like chemistry, biology, medicine, and environmental science, where precise shifts in acidity can have major consequences.
Here’s how the scale breaks down:
- pH < 7: The solution is acidic; it contains more H⁺ ions than hydroxide ions (OH⁻).
- pH = 7: The solution is neutral, H⁺ and OH⁻ are balanced. This is typical of distilled water.
- pH > 7: The solution is basic; OH⁻ ions outnumber H⁺ ions.
For example, a solution with a hydrogen ion concentration of 1 × 10⁻⁴ M has a pH of 4, making it mildly acidic.
Today’s digital pH calculators and sensors simplify this process by automatically analyzing [H⁺], [OH⁻], and even pOH to provide an accurate value and position your result properly on the scale.
pH Formula and Calculation
The pH of a solution is determined by the concentration of hydrogen ions [H+][H⁺][H+] in that solution. The standard pH formula is:
This formula is logarithmic, meaning every whole number change represents a tenfold difference in acidity. For example:
That tells you the solution is acidic.
To calculate pH, you need to know the molarity (mol/L) of the acid or base, either directly or from mass and volume. The calculator handles this behind the scenes, whether you input:
- [H⁺] directly
- Acid/base concentration
- Mass and volume of solute
If you’re working with strong acids like HCl, which fully dissociate, the calculation is straightforward. For weak acids and bases, the tool also incorporates Ka or Kb values to determine the equilibrium [H⁺] before calculating the pH.
I used to struggle with chemistry until I finally understood that pH is just a log equation. Once I realised that calculating pH was about taking the negative log of the hydrogen ion concentration, it suddenly clicked. I even made a quick-reference card during exams, which saved me more than once.
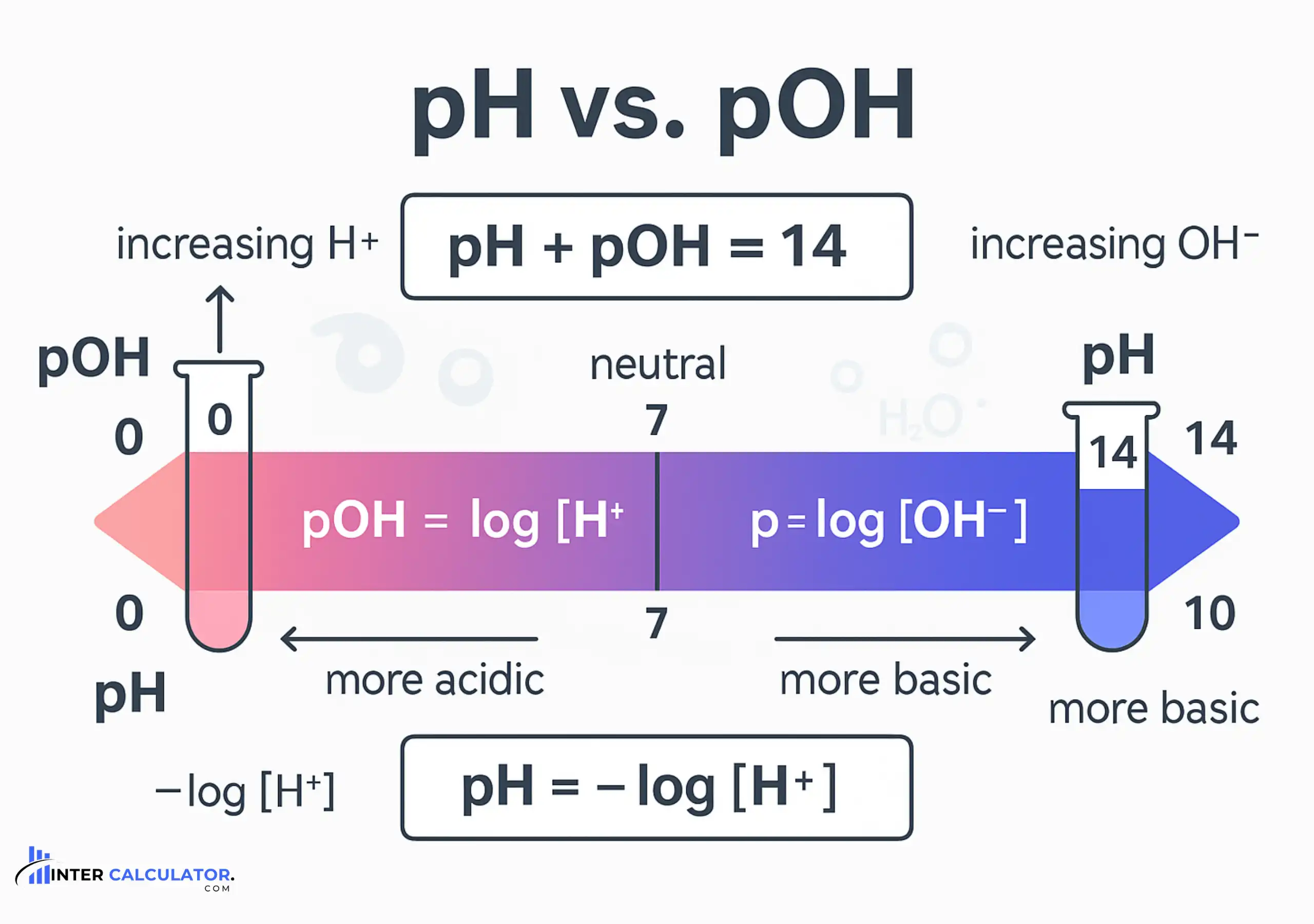
pOH and the pH Relationship
While pH measures the acidity of a solution by indicating its hydrogen ion concentration [H+][H⁺][H+], pOH does the same for basicity, reflecting the hydroxide ion concentration [OH−][OH⁻][OH−].
The pOH Formula
The formula for pOH mirrors that of pH:
This means the lower the pOH, the higher the concentration of hydroxide ions, indicating a stronger base. For example:
That value suggests the solution is moderately basic.
pH + pOH = 14
At 25°C, pH and pOH are mathematically linked by this universal relationship:
pH + pOH = 14
This equation is derived from the autoionization of water, where water molecules naturally dissociate into [H+][H⁺][H+] and [OH−][OH⁻][OH−] ions:
H₂O ⇌ H⁺ + OH⁻
The equilibrium constant for this reaction is known as Kw, and it equals:
Kw = [H⁺] × [OH⁻] = 1 × 10⁻¹⁴
This constant allows you to calculate any one value, pH, pOH, [H+][H⁺][H+], or [OH−][OH⁻][OH−], as long as you have at least one of the others.
I still remember a lab session where one of my classmates confused pH with pOH, and we ended up mislabeling all the test tubes. It wasn’t a disaster, but it did throw off our results and taught me a simple lesson: always double-check that pH and pOH add up to 14. It’s easy to overlook when you’re in a rush, but it makes a big difference.
Real-World Use: Why It Matters
Understanding the pH-pOH relationship is essential in any environment where pH control is critical, like chemical manufacturing, environmental testing, water treatment, and biochemistry. For example:
- A solution with pH = 3 must have pOH = 11
- If you enter [OH⁻] into the calculator, it automatically computes both pOH and pH using this relationship
Whether you’re working with acids, bases, or just calculating from ion concentrations, this tool simplifies the math and ensures accuracy by applying these scientific constants in the background.
How to Calculate pH Manually, Step-by-Step Solution
Whether you’re dealing with a strong acid, a weak base, or an unknown solution, the process of calculating pH follows a clear scientific method. Here’s a step-by-step breakdown on how to calculate pH accurately:
Step 1: Identify Your Known Values
Determine what you have:
- Molar concentration (mol/L) of an acid or base
- Mass and volume of a substance
- [H⁺] or [OH⁻] ion concentration
- Ka or Kb value (for weak acids/bases)
Choose the correct input type in the pH calculator to match your data.
Step 2: Convert Inputs to Molarity (if needed)
If you have mass and volume instead of molarity, convert using:
Our calculator does this automatically when you select “mass and volume” as input.
Step 3: Apply the pH or pOH Formula
Once you have molarity or ion concentration, use:
- For [H⁺] known:
- For [OH⁻] known:
pOH = –log₁₀[OH⁻], then pH = 14 – pOH
These formulas rely on the logarithmic scale, where every unit change reflects a 10× difference in ion concentration.
Step 4: Use Ka or Kb for Weak Acids/Bases
If you’re calculating the pH of a weak acid or base, and only know the concentration and Ka/Kb, you’ll need to use the ICE table method or allow the calculator to do it for you.
The tool uses this formula for weak acids:
[H⁺] = √(Ka × C), then
calculates:
pH = –log₁₀[H⁺]
Step 5: Confirm Results with Relationships
- Check if pH + pOH = 14
- Verify [H⁺] × [OH⁻] = 1 × 10⁻¹⁴ at 25°C
- Use pH to classify the solution:
- pH < 7: Acidic
- pH = 7: Neutral
- pH > 7: Basic
Examples of Strong and Weak Acids and Bases
Understanding whether a substance is a strong or weak acid/base is crucial for accurate pH calculation. This affects how completely the substance dissociates in water, which in turn influences [H⁺], [OH⁻], and ultimately the pH.
Strong Acids
Strong acids fully dissociate in water, releasing all of their hydrogen ions [H+][H⁺][H+]. This leads to a high concentration of H⁺, and therefore, a low pH.
Common examples:
- Hydrochloric acid (HCl)
- Nitric acid (HNO₃)
- Sulfuric acid (H₂SO₄)
- Hydrobromic acid (HBr)
- Perchloric acid (HClO₄)
- Hydroiodic acid (HI)
Example: A 0.01 M solution of HCl has a pH close to 2, because all HCl molecules release H⁺.
Weak Acids
Weak acids only partially dissociate in water. This means not all molecules release H⁺ ions, making the pH higher than that of a strong acid at the same concentration.
Common examples:
- Acetic acid (CH₃COOH)
- Formic acid (HCOOH)
- Citric acid
- Carbonic acid (H₂CO₃)
- Phosphoric acid (H₃PO₄)
Each weak acid has a Ka value (acid dissociation constant) that describes how strongly it dissociates. The pH calculator uses this Ka value for precise weak acid calculations.
Strong Bases
Strong bases fully dissociate in water to release hydroxide ions [OH−][OH⁻][OH−], resulting in a very high pH.
Common examples:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)₂)
- Barium hydroxide (Ba(OH)₂)
- Lithium hydroxide (LiOH)
Example: A 0.01 M NaOH solution gives a pH around 12, due to complete OH⁻ release.
Weak Bases
Weak bases only partially dissociate and produce fewer OH⁻ ions, making the pH closer to neutral.
Common examples:
- Ammonia (NH₃)
- Methylamine (CH₃NH₂)
- Aniline (C₆H₅NH₂)
- Pyridine (C₅H₅N)
Like weak acids, weak bases have a Kb value (base dissociation constant) that determines their dissociation strength. The calculator allows for custom base input, including Kb values, for accurate pH prediction.
By identifying your compound as strong or weak, the tool applies the correct dissociation logic, either assuming full ionization or calculating from Ka/Kb equilibrium, giving you a precise pH result every time.
Why We Built This Calculator
At Intercalculator, we believe that science should feel accessible, not intimidating. We created this pH calculator to make complex chemical calculations easier for students, professionals, and anyone curious about how acidity and alkalinity really work.
Too often, we’ve seen people struggle with logarithmic formulas or second-guess lab results because they weren’t confident in the math. Whether you’re studying for an exam, testing water quality, or working in a lab, we wanted to offer a tool that takes out the guesswork, while still helping you understand the “why” behind the number.
Personally, I remember spending way too much time trying to double-check my pH calculations during a water chemistry project. Every small mistake in the equation could throw everything off. That experience sparked the idea behind Intercalculator: why not build a tool that’s fast, accurate, and still educational?
So we built this calculator with simplicity in mind:
- You can input [H⁺], [OH⁻], pOH, or pH, and get everything else instantly.
- It uses trusted scientific constants and relationships like pH + pOH = 14 and Kw = 1 × 10⁻¹⁴.
- And best of all, it helps you learn as you go.
This isn’t just a plug-and-play tool; it’s a learning companion. With Intercalculator, you’re not just getting answers, you’re gaining clarity.
Final Thoughts
Calculating the pH of a solution doesn’t have to be complex. With this advanced pH calculator, you can determine acidity, basicity, and ion concentrations instantly, whether you’re working with strong acids, weak bases, or custom compounds. From direct [H⁺] input to full equilibrium calculations using Ka or Kb, this tool adapts to your needs with precision.
By understanding the pH scale, the role of pOH, and the step-by-step process behind each calculation, you can confidently analyze chemical solutions in labs, classrooms, or real-world applications. Fast, accurate, and scientifically sound, this pH calculator is your go-to resource for every acid-base challenge.
Frequently Asked Questions
Got questions? Our FAQs cover common topics about how our tools work, tips for accurate calculations, and guidance on using InterCalculator for everyday money decisions.
What is a pH Calculator used for?
A pH Calculator helps determine the acidity or alkalinity of a solution based on the hydrogen ion concentration. It’s widely used in chemistry, biology, environmental science, and even the food industry.
What is the pH scale and how does it work?
The pH scale ranges from 0 to 14. A pH below 7 indicates acidity, a pH of 7 is neutral, and a pH above 7 indicates alkalinity (basicity). Each unit represents a tenfold change in acidity or basicity.
Can the calculator handle both strong and weak acids/bases?
Yes, but the method differs. For strong acids and bases, pH can be calculated directly. For weak acids and bases, the calculation involves equilibrium constants (Ka or Kb) and requires more complex formulas.
Why is pH important in real life?
pH affects chemical reactions, biological processes, and product safety. It’s critical in areas like agriculture, water treatment, medicine, cosmetics, and food production.
Does the calculator provide exact lab results?
No, it gives accurate theoretical values based on the inputs you provide. For precise measurements, especially in professional or safety-critical settings, a pH meter or test kit should be used.
This calculator was created by the InterCalculator Editorial Team, led by Haris Farooq (Formula & Development). Our team specializes in formula research, calculator logic, and technical development, ensuring each tool is accurate, fast, and easy to use.
View Editorial Team →Before publishing, every calculator goes through the InterCalculator Accuracy Review Process. For the pH Calculator, we verify formulas against trusted chemical and scientific standards for pH measurement. We test results across multiple acidic, neutral, and alkaline solution scenarios to ensure precise and consistent outputs. All calculations are reviewed with an experienced chemist to confirm accuracy, clarity, and reliability.
View Process →